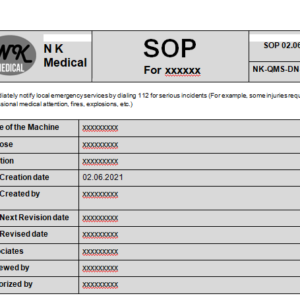
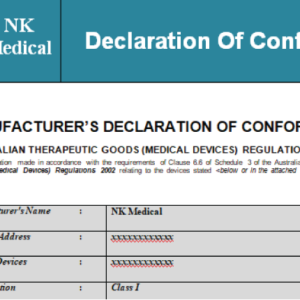
Your content goes here. Edit or remove this text inline or in the module Content settings. You can...
Grow Your Business With MDR Compliance
I help Medical Device companies – from startups to enterprises- comply with EU MDR 2017/745 and get ISO 13485 certified. Get assistance with 510K clearance, Technical files, and registration with regulatory bodies.
Our Services
Consultation
Understand MDR
ISO 13485
510K Clearance
MD Technical File
RA Registration
EU MDR 2017/745
The EU MDR 2017/745, or European Union Medical Device Regulation, is a comprehensive set of regulations governing the production and distribution of medical devices within the European Union. Enforced since May 26, 2021, it replaced the earlier Medical Device Directive. The primary objective of the EU MDR is to elevate the safety and efficacy standards of medical devices.
Classification system
Increased security
Post-Market Surveillance
UDI
Clinical Evaluation
Transparency & Traceability

Latest Posts
Specialist in Medical Device Registration
Your content goes here. Edit or remove this text inline or in the module Content settings. You can...
Front and Backend Web Development
Your content goes here. Edit or remove this text inline or in the module Content settings. You can...
Products on Sale
Showing 9–15 of 15 results
Contact US

Let's Discuss together
We are here for you 24/7. Please send us your thoughts and requirements.
A Team of Experts Behind the Wheel
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.

Shola Akinlade
CEO, Founder
Tani Tondi
COO, Cofounder

Thomas Boliva
Lead Designer

Amanda Kline
Head of Sales